May 26th 2020 is rapidly approaching and we still awaits high impact guidelines. However, this does not mean that you can sit around and wait, doing nothing. In an ideal world you should already have formulated a comprehensive step-by-step transfer plan and be implementing it right now.
We highly recommend that the MDR transition is performed as a defined project, and not a separate activity for the regulatory department. To ensure a successful project it requires a structured approach. It also needs to involve most of the operations within the company as well as a clear management commitment.
Transition to the MDR will include changes of the product documentation and QMS. Furthermore, the MDR has clear emphasis on lifecycle-based processes (including the entire supply chain) and continuous follow-up through planned risk-based PMS and PMCF. Hence, continuous compliance will require changes in the way every organization work and handle their product documentation.
Which step should the MDR transfer project include?
To start with qualification and classification is key because it impacts the regulatory requirements for the medical device, as well as the approval route and its associated costs. Furthermore, it provides crucial input to your transfer strategy as it determines whether your product e.g. qualifies under the extended transition period provided by corrigendum II.
Additionally, it is important to realize that the MDR not only will result in re-certification and up-classification of existing medical devices. Its scope will also include products that are not currently qualified as medical devices or accessories under the MDD, e.g. non-medical devices with a risk profile similar to medical devices such as cosmetic implants and contact lenses.
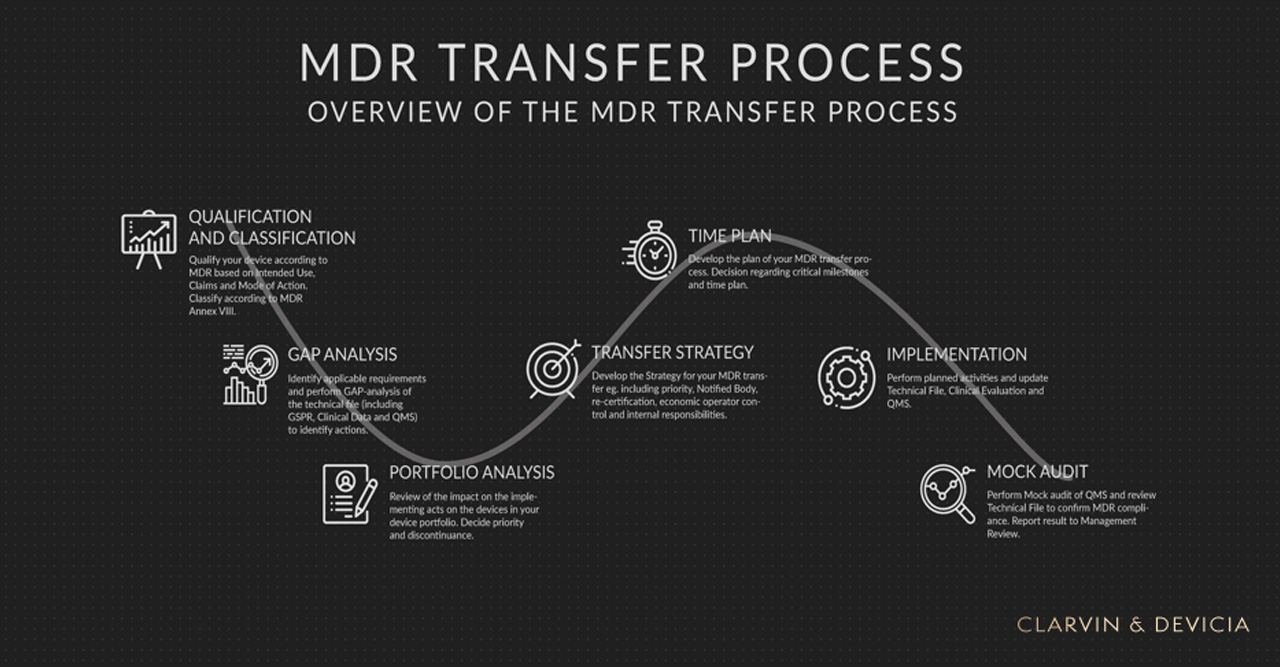
One of the buzz words during the recent years have been GAP analysis. This may not be our favorite word, but the fact is that GAP analyzes are key in the MDR transfer process. Thus, it is as part of the GAP analysis we will identify the requirements, i.e. what we need to do to fulfill the relevant requirements of the MDR, and how far away we are with our current documentation. Based on the result of the GAP analysis we can then shape our project and define our transfer strategy as well as related activities and time plans.[/vc_column_text][/vc_column][/vc_row][vc_row][vc_column][vc_single_image image=”3212″ img_size=”full” add_caption=”yes” parallax_scroll=”no”][vc_column_text text_larger=”no”]Figure 1 provides a schematic overview on how we suggest that you structure your MDR transfer process.[/vc_column_text][vc_column_text text_larger=”no”]Is it enough with one GAP analysis?
We recommend that you conduct several GAP analyzes as part of your MDR transfer project. This to cover all relevant aspects such as:
· General Safety and Performance Requirements (GSPR)
· Clinical data
· Quality Management System
· Technical File
Some of the GAP analyzes needs to be performed on a product per product basis whilst others are more general and can be performed on a company organization level.
How to ensure that you have sufficient clinical data?
There are several reasons for the increased requirements on clinical data. In general, the purpose of the MDR is to ensure a high-level of health and safety protection for EU citizens. Additionally, the MDR is specifically emphasizing that the requirements for clinical data and clinical evaluation (which together constitute the clinical evidence) become more stringent.
Clinical evaluation is not a new requirement. Already with the 4th revision of MEDDEV 2.7/1 (published in June 2016) the requirements for clinical evaluation were tightened, and in fact they provided a bridge to the requirements that we now can see in the MDR.
As the requirements for clinical data has increased, the results from clinical investigations and/or PMCF studies that already has been performed or is currently ongoing will be highly valuable as part of your clinical evaluation process. But this is only if they have been collected in compliance with ISO 14155. Clinical data collected in clinical investigations that lack sufficient ISO 14155 compliance are at risk of providing insufficient clinical evidence, and that is something we have noticed the notified bodies are scrutinizing more in-depth today.
One common question we receive is; “Do we have sufficient clinical evidence for MDR compliance?”
The answer to that question really depends on the current quality and quantity of your clinical evidence, meaning your clinical data and clinical evaluation documentation. Our recommendation is to assess this by conducting a GAP analysis.
Does the information above feel like an overwhelming task?
If your answer to the question above is yes, you are not alone. Several medical device manufacturers feel that the MDR transfer is an overwhelming task, especially if they have several products or limited internal resources. We can provide you with adequate support and help you develop a sustainable strategy, not only for the MDR transfer but also for staying on the market and gain acceptance for your product.